Principle of UV Absorber & UV Stabilizer
When polymer materials are used outdoors, it will be photodegradation by ultraviolet light (UV-light): the polymer absorbs ultraviolet energy to break the molecular bond to form a radical, and the radical reacts with oxygen to form a peroxy radical, thereby destroying the structure of the polymer itself and generating more free radicals. It destroys the stability of the polymer and causes discoloration and deterioration.
In order to avoid such a situation, it is necessary to add a light stabilizer to the polymer. The light stabilizer can be classified into three categories according to the principle:
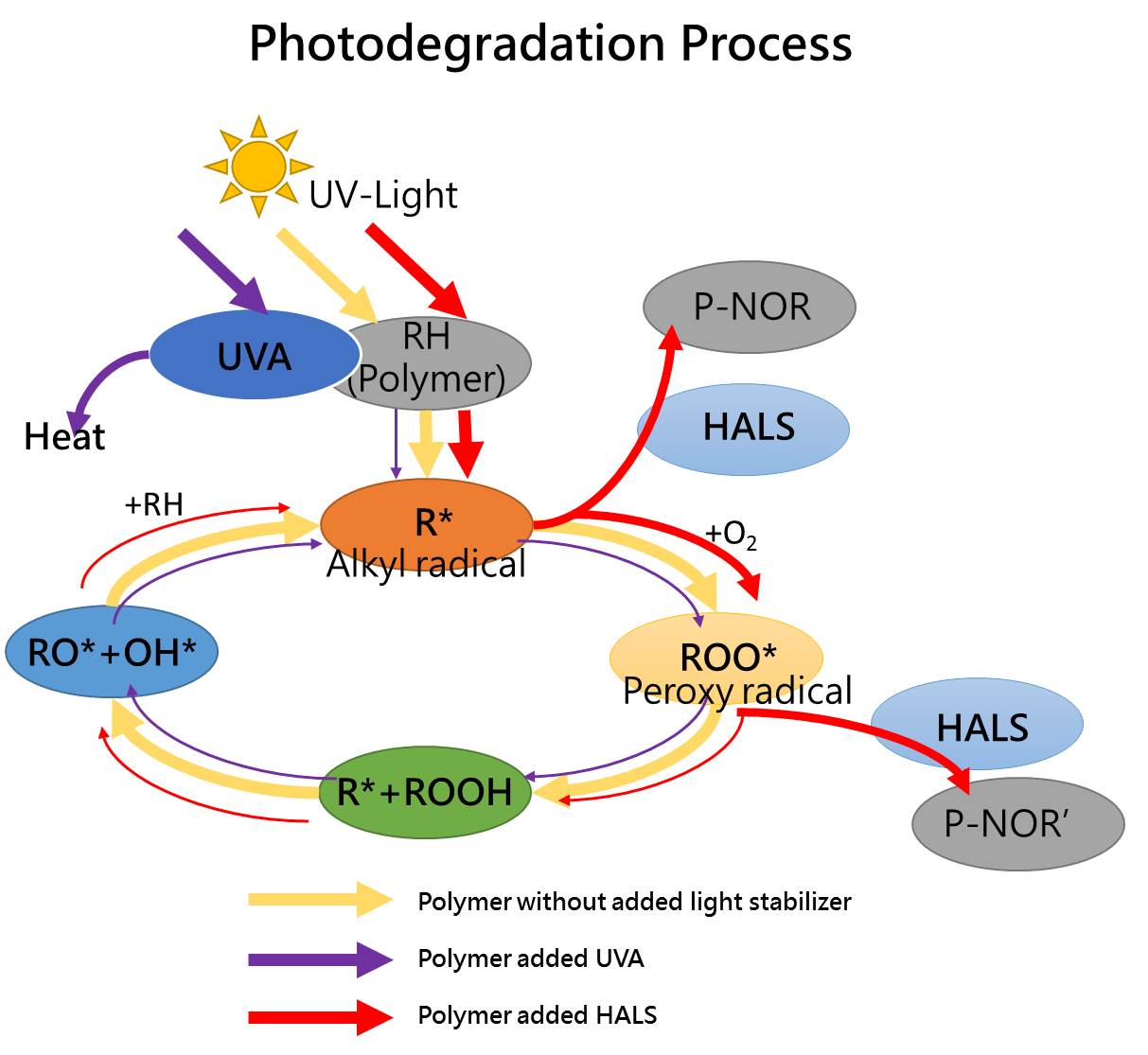
The ultraviolet light absorber incorporated with the polymer competes with the polymer to absorb ultraviolet rays, reduces the ultraviolet light absorbed by the polymer, and reducing the occurrence of photodegradation and prolonging the life of the polymer.
The light stabilizer has the ability to capture free radicals, which can effectively avoid the degradation phenomenon, and the free radicals will be trapped to move back to a stable state, and will not react with the substances to form more free radicals, thereby effectively avoiding the destruction of the material structure. To reduce the occurrence of degradation
Using special design structure and exclusive formula, it can effectively prevent the polymer from absorbing ultraviolet rays, inhibit the phenomenon of photodecomposition, achieve long-term effective light stabilizer effect, and protect products from UV light.